Abstract
Allogeneic off-the-shelf cell therapies offer distinct advantages over conventional autologous cell therapies in terms of scaled manufacturing, on-demand availability and optimization of cellular starting material. A unique consideration in the use of allogeneic cell therapies is the potential for immune cell-mediated recognition of the allogeneic cell product by the patient's immune system. CAR T-cell therapies are commonly combined with conditioning chemotherapies that suppress a patient's immune system, creating a suitable window of activity to elicit clinical response. However, protracted lympho-conditioning also affects immune reconstitution and can negatively impact the rate of infection. Alternative approaches to prevent allorejection may therefore help to enhance the efficacy of the therapy while preserving the immune system of the patient.
Elimination of cell-surface human leukocyte antigen (HLA) molecule expression by genetic knockout (KO) has long been known to abrogate T-cell reactivity. However, loss of class I HLA elicits NK cell-mediated recognition and clearance, and therefore must be combined with other immune-modulating strategies to limit host NK cell reactivity. Allogeneic models combining class I HLA deletion with NK cell inhibitory molecules, such as HLA-E and CD47, have been shown to abrogate NK cell reactivity in mouse models. However, HLA-E is the canonical activator of NKG2C, a dominant activating receptor found on human NK cells. Likewise, the expression of signal regulatory protein alpha (SIRPα), the major interactor for CD47, is mostly restricted to macrophages and dendritic cells and not human NK cells, and the observed effects of this immune-modulating strategy in the mouse system may only offer partial or incomplete immune evasion in the human system.
In this study, we provide details of a bona fide off-the-shelf strategy where iPSC-derived NK (iNK) cell therapy is multiplexed engineered with a novel combination of immune-evasion modalities; beta 2 microgobulin (B2M) KO to prevent CD8 T-cell mediated rejection; class II transactivator (CIITA) KO to prevent CD4 T-cell mediated rejection; and CD38 KO to enable combination with anti-CD38 mAbs, which can be administered to deplete host alloreactive lymphocytes, including both NK and T cells. In vitro mixed lymphocyte reaction (MLR) data demonstrated that upon co-culture with allogeneic PBMCs, B2M KO iNK cells stimulated less T-cell activation than their B2M sufficient counterparts as evidenced by reduced CD38, 41BB, and CD25 levels on T cells. Additionally, B2M KO iNK cells impaired T-cell expansion over the duration of co-culture, resulting in a 50% decrease in expansion at the peak of the control response. However, B2M KO iNK cells were depleted over time, suggesting activation of an NK cell "missing self" response by the peripheral blood NK (pbNK) cells.
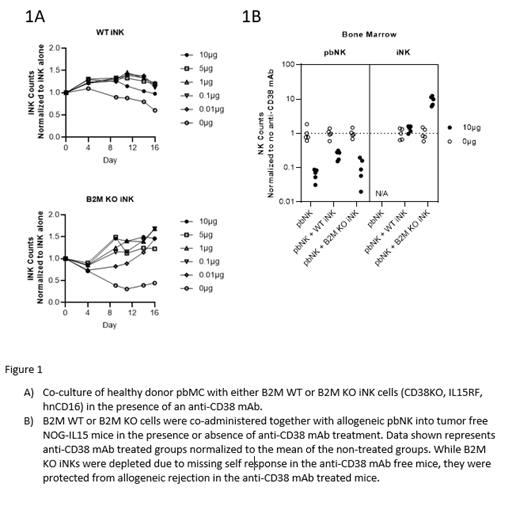
In contrast, when the assay was performed in the presence of anti-CD38 mAb, depletion of B2M KO iNK cells was blocked, and instead B2M KO iNK cell numbers increased by 3.5-fold, comparable to the iNK cell numbers found in the control arm (cultured without allogeneic PBMCs). Interestingly, pbNK cell numbers decreased, while T-cell activation and expansion remained lower than in B2M-sufficient MLR cultures. Furthermore, when B2M KO iNK cells were cocultured with tumor cells and anti-CD38 mAb in vitro, ADCC was comparable to the B2M sufficient cells, indicating uncompromised effector function. Finally, in vivo studies suggested that co-administration of anti-CD38 mAbs can significantly enhance the persistence of B2M KO iNK cells in the presence of allogeneic pbNK cells as seen in the spleen and bone marrow (Figure 1).
Together these data demonstrate that the combination of triple-gene knockout of CD38, B2M and CIITA with a CD38-targeting mAb is an effective strategy to avoid host immune rejection, and highlights the potential advantages of multiplexed engineered iPSCs to facilitate large-scale manufacture of complex engineered, off-the-shelf cellular therapies.
Williams: Fate Therapeutics: Current Employment. Malmberg: Merck: Research Funding; Vycellix: Consultancy; Fate Therapeutics: Consultancy, Research Funding. Lee: Fate Therapeutics, Inc.: Current Employment. Bjordahl: Fate Therapeutics: Current Employment. Valamehr: Fate Therapeutics, Inc.: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal